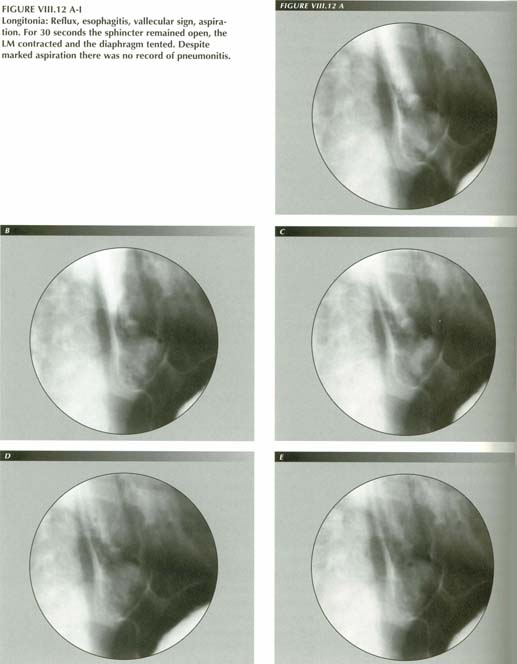
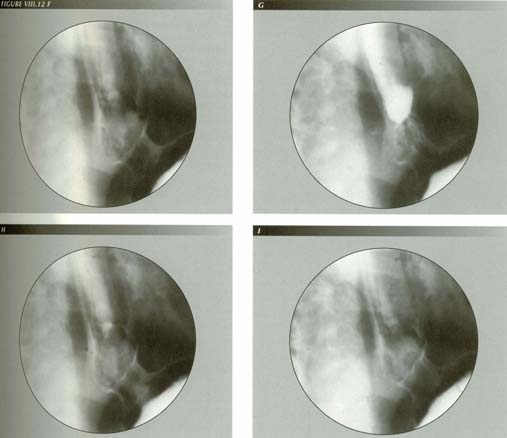
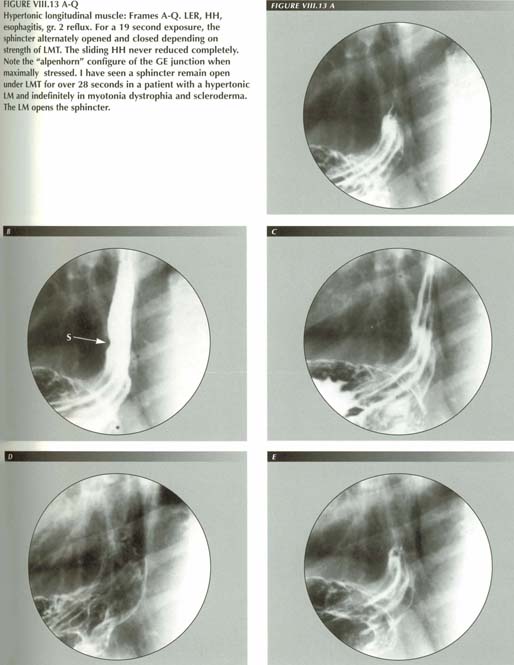
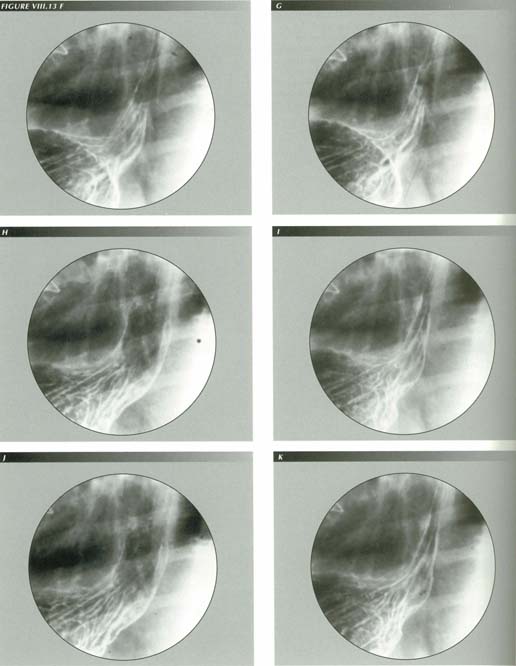
Longitudinal muscle contraction
It is important to recognize that the longitudinal muscle (LM) is completely
invisible to intraluminal manometers, transducers and balloons. Unlike
contraction of the circular muscle or sphincter, LM contraction does not affect
intraluminal pressure. For that reason, the LM is the "lost muscle" of the esophagus.
This circumstance explains the paucity of information about the function of
the LM in the vast literature of the esophagus. It is not even in the index
of Bockus. Castell(1) does not mention it after
page 11.Standard reference works, textbooks and even monographs(2),(3),(4),(5)
restrict their discussion of the LM to a description of its anatomy. Otherwise
minutely detailed physiologic and radiologic descriptions of the process of
deglutition,(6) ignore the LM. A monograph on
disorders of esophageal motility(7) does not
mention the longitudinal muscle in that connection. In his review of the recent
literature, Diamant found no studies attempting to distinguish between the physiological
characteristics of longitudinal and circular muscle fibers. An extended computer
search of the medical literature for the years 1966-94 retrieved no references
to the longitudinal muscle.
This creates unique problems and opportunities for the investigator. There
is little infrastructure on which to build and the reports that do shed light
on the action of the LM must often be reinterpreted.
LM anatomy
A surprising variation in LM anatomy exists among mammalian species. The dog
is considered a poor model because its muscle, instead of being arranged in
a separate inner circular layer and outer longitudinal layer, consists of two
helical sets of fibers. A similar arrangement is found in the cow, sheep, camel
and other species. Like humans, the cat and opossum have inner circular and
outer longitudinal layers and are so considered more suitable species for physiological
research. In teleosts (fish with boney skeletons) it is surprising to learn
that the order is reversed with the LM inside the circular.
The relative proportion of the total muscle mass in each layer is also variable.
In the cat, the LM is well developed. In the rat it is tenuous. (8)In
humans, more than 50% of the muscle mass of the esophagus is LM - the reverse
of the situation elsewhere in the gut.(9),(10)
| Type(11) | % CM | % LM |
| All striated | 4.2 1.5 | 5.7 .33 |
| Mixed | 34.5 2.4 | 41.1 3.3 |
| All smooth | 62.2 2.9 | 54.1 3.4 |
The preceding table, after Meyer & Castell,(12)
tabulates the percent of the total esophageal length the indicated muscle type
was present. Average length of 11 autopsy specimens of the esophagus was 22
2.3 cm. The table cannot be taken literally. I have reviewed sections taken
from the upper, middle and lower esophagus in 12 autopsy specimens. On any given
section, cells are cut transversely, longitudinally and obliquely. It would
take an elaborate statistical analysis to approximate the number of fibers in
each direction. The table ignores the fact that many fibers are cut obliquely
and are neither longitudinal nor circular. Such fibers must be spiral.
Since the work of Kaufmann et al.,(13)
the conventional view that the human esophagus is composed of an inner circular
and an outer longitudinal layer of muscle may require revision. These authors
describe spiral fibers running in all directions: clockwise up, clockwise down,
counterclockwise up and down. They appear to start from the adventitia and end
at the submucosa.
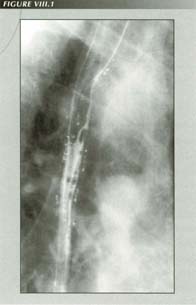
So-called “intramural diverticula:” The name is an oxymoron as if they are intramural, they are, by definition, not diverticula. The only thing they could be due to is barium in ectatic mucus glands, the glands that provide esophageal lubrication. [Case provided by O.Arthur Stiennon III]
In dogs and most rodents, the esophagus contains chiefly or only striated
muscle. In cats, striated muscle makes up all but the distal 1/4th.(15)
Pelot(16) as well as Netter and Mitchell(17)
state that the LM is thicker than the circular muscle in man, a relation which
is unique to the esophagus and the reverse of the rest of the gut where the
circular muscle predominates. The proportion of LM to CM varies not only longitudinally
but axially. Near its origin from the cricoid cartilage the LM is mainly massed
in two thick bundles posterolaterally. The average thickness of the esophageal
wall as measured by ultrasound is 2.6 mm.(18)
Earlam(19) states that smooth muscle cells
form a syncytium. They transmit electrical signals via a low resistance connection
called a nexus so small it can only be seen with the electron microscope. There
is no difference between circular and long smooth muscle in this respect.
The median thickness of the mucosa is .2mm(20) The organ functions exclusively as a conduit. Unlike the rest of the gut, no digestive enzymes are secreted here. Its only glands are the lubricating, mucus-producing glands. The stratified squamous epithelium best resists erosion.
The phrenoesophageal ligament (PEL), which attaches the esophagus to the diaphragm
is extraordinarily elastic and seems to have been designed to buffer the force
of LMC. Hayward(21) noted that when the PEL
was cut, its proximal portion retracted into the adventitia. Groszek and Matysiak(22)
state that it does not have the histologic structure of a true ligament (thick
collagenic fibers) but is composed of collagenic fibers with a rich admixture
of elastic fibers which begin to appear antenatally and show increasing prominence
into young adult life. They diminish in number with advancing age. A maximal
sliding HH is 8 cm on films. Allowing for 30% enlargement by the divergent beam,
this is an esophageal shortening of 5.6 cm or 25% of its length without rupture
of the PEL. This remarkable elasticity serves an essential function in deglutition.
The vagus trunk in the thorax is mainly sensory (afferent fibers) and not motor.
It is estimated that there are only 3000 efferent fibers in the vagus (for 5,000,000
ganglion cells in the gut.)
It is important to remember that the esophagus is merely a specialized segment of gut. The most significant difference between the esophagus and the rest of the gut is its relatively fixed length. Firmly secured to the cricoid cartilage above and, via the phrenoesophageal ligament (PEL), to a less mobile portion of the diaphragm below, its ability to shorten is limited to the elasticity of those attachments and the position of the diaphragm. As respiration is inhibited during deglutition, the diaphragm is often stationary when the LM is active.
LM physiology
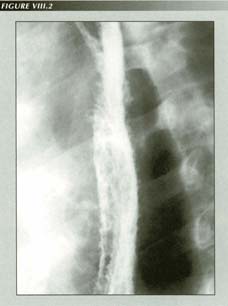
Candita infection with AIDS: Note the elargement of the mucus glands.
In lower animals at least, peristalsis can occur even though the organ is denervated.
This suggests myenteric or myogenic transmission and control of peristalsis.
Yet the experiments of Janssens(25) show that
a central program controls peristalsis. A peristaltic wave crossed the transected
esophagus whether or not it was re-anastomosed. Balloon distention of the distal
segment after transection would incite peristalsis in the proximal segment.
Yet without vagal input the intramural plexus can produce peristalsis independently
- an apparent example of distributed processing. In this the esophagus is similar
to the jejunum, a free graft of which to replace a resected cervical esophagus
continued to show independent migrating muscular contractions.(26)
Stimulating the cut end of the vagus will reduce lower esophageal sphincter
pressure (LESP) but this effect may not be primarily on the sphincter as such
stimulation also causes LM contraction. Stimulation of the dorsal motor nucleus
of the vagus or nucleus ambiguus will also lower sphincter pressure if the vagus
is intact.(27) Oxygen dependence of the lower
esophageal sphincter (LES) muscle of the opossum has suggested a clue to reflux
in anemias.
In the opossum,(28) the LM contracts throughout the duration of electrical stimulation. The CM, on the other hand, begins contracting after the stimulus - either electrical or stretching - is turned off.
Normal physiologic pressure measurements
| Level | Pressure |
| Pharynx | + 0 mm Hg |
| UE sphincter | +100 mm Hg |
| Body | - 5 mm Hg |
| LE sphincter | + 20 mm Hg |
| Stomach | + 5 mm Hg |
LM power
The LM has a contractile power so great it can tear loose from its moorings
at the hypopharynx and at the diaphragm.(29)
LMC is the only conceivable explanation for tearing the stitches out of a fundoplication.
It may even pull the plicated stomach through itself and/or through the hiatus
in failed Nissen fundoplications.(30) Either
by attrition or brute force, it can rupture the phrenoesophageal ligament. Its
power is such that it can pull the stomach through a constricting Angelchik
prosthesis.(31),(32),(33)
Although the force of LM contraction (LMC) has not been reported in man, an
estimate can be derived from measurements of intraesophageal pressure. Presumably,
a LM fiber can exert at least the contractile tension of a fiber of circular
muscle. The LM mass, however, is greater. Given that the CM can produce manometric
pressures of 200 mm Hg on a catheter of, say, 4 mm radius we can apply Laplace's
law(34) to arrive at the wall tension.
Laplace's law states that p = t/r, where t = wall tension, p = intraluminal
pressure and r = radius. Inserting these values in the formula gives:
200 mm Hg = t/ 4 mm, or t = 800 mm2Hg and dividing by the CM thickness
of 1 mm gives 800 mm Hg. But 1 mm Hg = .01934 lb/inch2. Substituing
in the above formula yields the considerable tension of 15.7 lb per square inch
for the CM. Maximum LM tension should be at least this great - enough to create
havoc at its attachments. In abnormal cases ("nutcracker esophagus") 350 mm
Hg has been recorded - equivalent to 27.6 lb/in2.
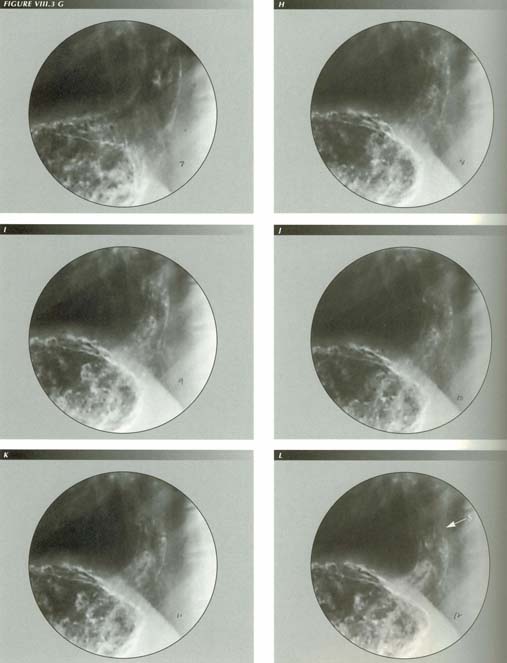
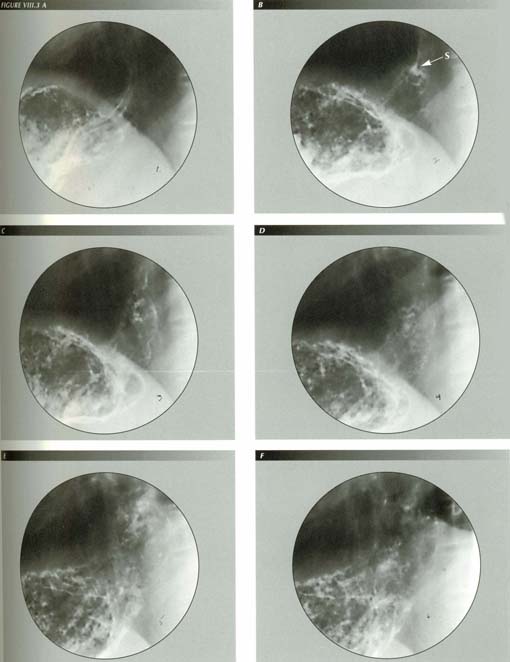
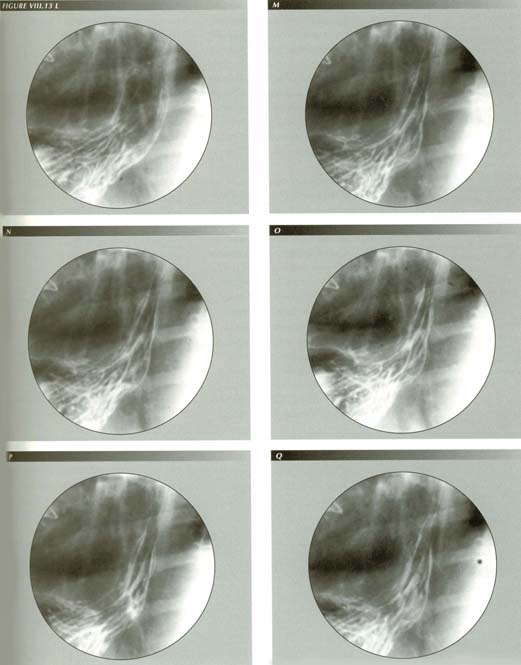
FIGURE VIII.3 A-LLM power and is effects: This sequence illustrates both the remarkable power of LM contraction and the equally remarkable elasticity of the PEL. In frame A there is just a nubbin of stomach above the diaphragm. The diaphragm is sharp. In frames B-F, made during a belch, the stomach is relentlessly transtracted through the hiatus 7.6 cm as the esophagus shortens 35% of its length. The diaphragm itself is tented and the hiatus stretched to its widest extent (frame E). LM contraction, as here, widens the hiatus thus affecting measurements of hiatal squeeze. Note the wide open sphincter and the alpenhorn sign as the LM relaxes slightly in frame G. Further relaxation closes the sphincter.
Torrance could produce vomiting by stimulating the central end of
the transacted vagus. Apomorphine potentiated this stimulus. A strong LMC was
the penultimate event of vomiting. He also found that vagal stimulation produced
gastroesophageal reflux from a water distended stomach. Reflux was accompanied
by minimal increase in intragastric pressure.
Torrance concluded reflux was ". . . . due to a mechanical effect on the oesophago-gastric
junction and not merely due to inhibition of the circular smooth muscle fibers
of the distal esophagus." He based this conclusion on the following:
Curare(37) completely eliminated reflux after
vagal stimulation,
Mechanical traction on the esophagus produced immediate regurgitation comparable
to LMC contraction.
Sympathectomy 6 weeks prior to vagal stimulation had no effect.
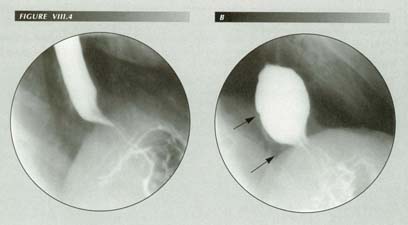
“Gas bloat” with a slipped Nissen 10 years after the operation: A Nissen fundoplication slips because LM tension pulls the esophagus through the encircling cuff of stomach, everting the latter in the process and resulting in the complex topology seen here. The esophagus can pass through the hiatus as can a portion of the fundus that is drawn out into a tube (B). The ora serrata (arrow) is 2.5 cm above the diaphragm (arrow), but the stomach bulk must stay behind, forming the mass of tissue which now separates the fundus from the diagphragm. No more dramatic illustration of the power of LMC can be found than its ability to achieve this complication. Because of it, the LM now has a lower purchase on the stomach and need shorten the esophagus correspondingly less to produce the gas/bloat sensation or, from another point of view, the same degree of LMC will produce worse symptoms. Hence the frequency of gas/bloat after this procedure. Extreme LM tension trasmitted to the diaphragm can also produce nausea, a symptom this patient has experienced for 10 years. He also has nocturnal reflux which he believes i affecting his lungs. He has lost 7 teeth in the last 3 years.
The effect of vagal stimulation on LMC has been studied quantitatively in dogs
by Edwards(38) who measured an elevation of
the GE junction of 2.2 cm [~ 25% shortening] on stimulation of one vagus nerve
and 2.4 cm on stimulation of both. The contraction was described as "violent"
with a nearly vertical kymograph tracing. Displacement was so abrupt that simultaneous
measurement of LES pressure was impossible. This velocity of contraction is
seen in human subjects during vomiting and must be unique for an organ that,
unlike the dog esophagus, is largely smooth muscle.
LMC was abolished by d-tubocararine but not by l-hyoscine and, in agreement
with Torrance and Johnson, Edwards believed the striated muscle was responsible
for the shortening. In the dog esophagus, which is entirely striated muscle,
abolition of contraction by curare is to be expected. The result should not
be extrapolated to man.
Edwards could abolish most LM activity by stripping one or both vagi from
the esophageal musculature. Tacitly recognizing that LMC could cause HH, he
suggested that selective vagotomy might be employed as an adjunct to anatomical
HH repairs to prevent recurrence.
Matthews, Thorpe and Little(39) stimulated
a single vagus at thoracotomy in several groups of patients. The control group,
that had no mediastinal disease, all showed a "brisk" response - defined as
1.5 cm or more of shortening in 1 to 2 seconds - to stimulation of either nerve.
On the average, patients with HH showed a diminished response to vagal stimulation
[perhaps because the esophagus was already shortened]. Those with achalasia,
as might be expected, showed no response to vagal stimulation. The less profound
contractions seen may well be attributable to the fact that these patients were
under general anesthesia.
Pharmacology
Neurohumoral agents affecting LESP
(After Ouyang-Cohen)
| Increases LESP | Decreases LESP |
| Acetylcholine | Beta-adrenergic agonist |
| Alpha-adrenergic agonist | Dopamine |
| Histamine (H1-receptor) | Histamine (H2-receptor) |
| Serotonin (neutral receptor) | Serotonin (neutral and muscle receptor) |
| Gastrin | Vasoactive intestinal peptide (VIP) |
| Substance P | Progesterone |
| Methionine enkephalin | Secretin |
| Bombesin | CCK-OP |
| Pancreatic polypeptide | Glucagon |
| Motilin | Gastric inhibitory polypeptide (GIP) |
| Bradykinin | |
| Cyclic AMP |
Of course, these effects are simply gleaned from the literature. In most cases it is not known whether they affect the sphincter directly or by way of LMC or both.
LES pressure is lowered by: 1.) Fat ingestion. 2.) The secretin "family" (VIP,
gastric inhibitory peptide, glucagon) - especially VIP (vasoactive intestinal
peptide) 3.) Progesterone - the probable cause of heartburn of pregnancy.
Adrenalin (epinephrine) has two types of activity - alpha and beta. The main
beta activity of epinephrine is vasoconstriction. Propranolol blocks the beta
activity of adrenalin. Phentolamine is the alpha blocker.
Carminatives apparently lower the LES pressure. This class of products includes
peppermint, spearmint, onion, garlic, anise, caraway, cinnamon, cloves, dill,
fenner, rosemary and turpentine. Are all products of steam distillation. They
are said to promote easy belching.
Fluoroscopic recognition of LMC
Despite its lack of recognition, LM activity is apparent to the questing eye and can, on occasion, be dramatic. Because good landmarks define the lower end of the esophagus, fluoroscopic observation can establish that the LM contracts in at least four distinct ways. Moreover, all of these modes are purposeful, reproducible and fully integrated with the other muscular elements of the organ.
There are many signs of LM contraction (LMC).
1.) Orad displacement of lower esophageal landmarks including
The lower esophageal ring.
The notches of McLean
The sphincter itself.
The mucosal transition
2.) Tenting of the phreno-esophageal ligament, fundus or diaphragm which causes
a loss of sharpness of the latter.
3.) Retraction of the fundus through the diaphragm (HH).
4.) A trumpet shaped flaring of the mouth of the esophagus.
5.) "Tertiary contractions"
6.) On occasion, the esophagus, presumably because of left atrial enlargement,
can occupy two stable positions. In one of these the LM is relaxed and in the
other contracted. This so-called "wandering esophagus" provides an excellent,
although rarely encountered, opportunity to study the effects of LMC.
7.) With familiarity, one becomes constantly conscious of the state of the
LM merely by its gestalt - whether it meanders loosely among its neighboring
organs or is taut.
A cine camera is an invaluable aid in studying LM contraction because exposures can be made at a low frame rate (typically 7.5/sec) and subsequently viewed at a rapid rate (24 frames/sec). When LM contraction is accelerated, it becomes much more obvious, just as time lapse photography reveals the motion of an opening flower. Analysis of slow motion studies is less useful except for analysis of anatomical details. Exposure technique for spot films should be chosen to yield the broadest possible latitude as the usual "black and white" GI films will not show the PEL or the outer wall of the esophagus.
Although useful for some mucosal details and for demonstrating the LM response to gas distention, I have avoided double contrast studies as unphysiologic.
A conceptual model of the esophagus is a helpful guide to analysis of all components
of esophageal muscular activity because it focuses attention where the action
can be expected. Most esophageal functions are reproducible, so that the boolean
model gives a lead to what to be looking for next: from one vertex of the cube
there are only three things that can happen - one of the three components must
either contract if it is relaxed or relax if it is contracted.
The following descriptions were compiled from such directed observations.
In over 600 cases, cine strips of the phenomena under study were made for confirmation
or analysis.
Peristaltic LM contraction
Peristaltic LM contraction occurs when swallowing against resistance and as
a terminal cleanup during the ingestion of liquids. It is integral with the
peristaltic wave of the circular muscle. The LM is tensioned initially by the
voluntary act of swallowing. If one observes a marker - and a small Zenker's
diverticulum is occasionally a convenient natural landmark - he will see that
the initial act of swallowing is an upward excursion of the larynx and the mouth
of the esophagus to which it is attached. The excursion amounts to about the
height of one vertebral body and one interspace.
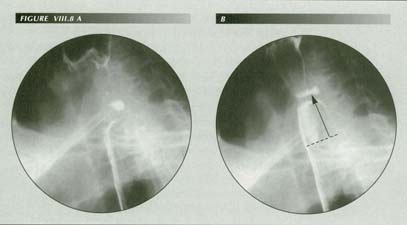
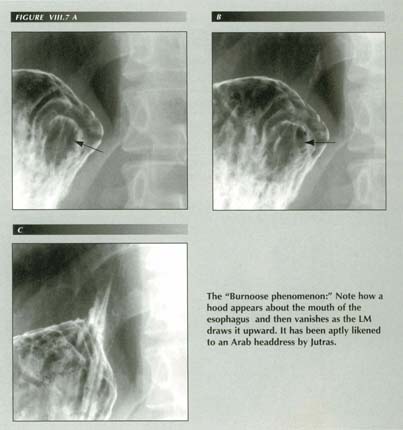
Symptomatic post-cricoid ring with Zenker’s diverticulum: CC: “Food sticks in throat.” Comparing these landmarks with the first rib or a cervical vertebrae shows they have an upward excursion of 2.8 cm at the outset of deglutition. This exerts an abrupt, forceful tug on the esophagus which, trasmitted to the PEL, may supply all the force needed to open the sphincter. the sharp tug may also trigger a stretch reflex causing LMC. As is invaiably the case with Zenker’s diverticula, the patient also had a HH. the association of the two is due to the circumstance that powerful LMC stretches or disrupts the esophageal attachment at the hypopharynx as well as at the diaphragm
More vigorous peristaltic LM contraction occurs during swallowing against resistance
as with ingestion of particulate food or during a Valsalva maneuver. As the
peristaltic wave moves aborally, the LM shortens in proportion to the distance
the cone of circular muscle contraction has moved toward the sphincter. Unlike
the circular muscle, that relaxes behind the peristaltic wave, the LM remains
contracted until the peristaltic wave is completed - as though circular
muscle activated LM in its path and "latched" it en passant.(40)(41)
From the characteristic manometer tracing, it is natural to think of a narrow
ring of CM contraction advancing toward the sphincter. Serial radiography, however,
shows that there are at least 5-7 cm of CM contracting at a time. Peristalsis
is not a migrating ring, but an advancing cone of CM contraction. By
the same token, a corresponding length of LM is excited. As the mouth of the
cone leads, wall contact is achieved only at the lagging apex of the cone. When
the leading edge of the cone of contraction approaches the sphincter region,
the cone becomes progressively shorter as its apex approaches its base and finally
vanishes leaving, at most, a nipple behind. Propulsion of the bolus, therefore,
is mediated by three things: 1.) Aboral migration of the cone of CM contraction,
2.) A decrease in the length of the cone and 3.) Shortening of the organ by
LMC.
As the peristaltic contraction cone forces a barium bolus ahead of it, the
simultaneous contraction of the LM, by shortening the esophagus, pulls the bolus
into the cone and opens and effaces the sphincter. The sphincter effacement
and orad excursion were also noted by Dodds, et al.(42)
in cats but the sphincter opening was attributed to passive stretching by the
bolus. Sphincter effacement is maximal when LM contraction produces maximal
shortening of the esophagus.
Once the sphincter opens, the esophagus and stomach form a common cavity thus
allowing the bolus to enter the stomach. Two events then happen so nearly simultaneously
that I have been unable to determine precedence with certainty:
1.) The LM relaxes.
2.) The peristaltic wave flows into the sphincter area and closes it.
Perhaps because of the structural differences of the sphincter region, the p-wave effectively becomes the sphincter when its closed end reaches the sphincter area. Thus, peristalsis stops at the sphincter and not at the end of the esophagus, i.e., the mucosal junction. This is easily observed and will account for some otherwise inexplicable findings in achalasia and its look-alikes. \
The aperistaltic segment
The peristaltic wave does not continue into or through the stomach. The stomach
has an independent pacemaker in the distal corpus discharging 3-4 times/minute.(43)
These discharges are not synchronized with deglutition, nor are they under voluntary
control as is deglutition. With the aid of the lower esophageal ring, or even
more frequently the notches of McLean, one can verify that the peristaltic wave
does not progress into or through a short esophageal zone .5 to 2.5 cm in length
extending from the lower margin of the sphincter above to the ring or notches
marking the mucosal transition below. This zone, which might be called the aperistaltic
segment, because it has neither sphincter tone nor peristaltic ability, acts
as an opening wedge for eructation of gas or gastric contents when elevated
by LM contraction.
The synchronized activities of LM, CM and sphincter during swallowing against
resistance are remarkably efficient in performing indispensable functions in
the simplest possible way. As the peristaltic wave advances, LM contraction
progresses concurrently with opening of the sphincter. Neither gas nor fluid
can reflux through the open sphincter because the advancing p-wave - in effect
a moving sphincter - prevents it. Reflux can not occur because the peristaltic
wave persists until it becomes the sphincter.
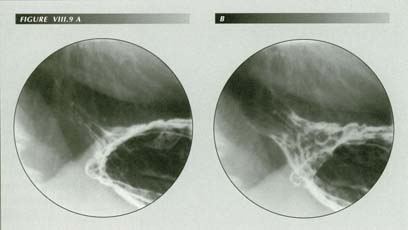
The longitudinal muscle opens the sphincter. Obvious sings of LMC in (B) correlate with the open sphincter. Note gastric mucosa above the diaphragm . The repeated stretching of the PE attachments over a lifetime will elongate or even rupture them.
Conceptually, one can think of the sphincter of having two components of tone:
a.) its baseline tone that even chemical denervation will not abolish and b.)
a supplementary component that it receives when the p-wave "merges" with it.
This would explain the sphincter closure even in esophagitis patients who have
lost their p-wave.
Christensen and Lund(44) found that circular
muscle stimulation (by distention) caused a much wider but undefined LM contraction.
This is consistent with the appearance of "latching" of the LM by the same neuronal
discharge that activates the CM. Apparently, when the LM is in peristaltic mode,
neural connections activate the LM adjacent to the advancing cone of CM contraction.
The results (also in the opossum) of Sugarbaker, et al. suggest that
LM contracts first and stays contracted longer.
One can also infer an inhibitory reflex originating in the sphincter area that, when activated by the arrival of the peristaltic wave, causes "unlatching" of the LM. Here, then, is a significant difference - one that would not have been expected a priori - between peristalsis in the two muscle layers:
In the CM, contraction is segmental with immediate relaxation both in front
of it and behind it.
In the LM, peristalsis causes incremental contraction involving the entire length of the esophagus. Relaxation occurs only after LM contraction reaches its maximum and peristaltic CM contraction reaches the sphincter.
The effect of LMC on the lower esophageal sphincter
The close temporal relation of sphincter closure and LM relaxation and vice
versa argues for a neurologic mechanism: arrival of the peristaltic wave at
the sphincter must signal a reflex relaxation of the LM. The relation of sphincter
opening and closing to LM contraction and relaxation is of the utmost importance
because this relationship explains many of the ills that afflict the esophagus.
The rule is:
LM contraction opens the sphincter.
All evidence points to sphincter release as the raison d'etre of
the LM. Just as closing the sphincter appears to reflexly deactivate the LM,
LM contraction reflexly deactivates the sphincter and also mechanically effaces
it. Timing of sphincter release high on the up-slope of the curve of LM contraction
is further evidence for LM release of the sphincter.
Elsewhere, I will show that whenever the sphincter is open in belching, reflux,
vomiting, etc., the LM is contracted. The contrapositive is also true: all conditions
that tension the esophagus - LM contraction, TEF repairs, cervical hyperextension,
myotonia, scleroderma, etc. - are associated with reflux.
Vector resolution of LMC force dilates the sphincter.
LMC and the trumpet GE junction: The force of LMC is resolved into 2 vector components both of which are well displayed here. a.) One component stretches the PEL, and b.) One opens the sphincter. Because these forces exist in 3 dimensions, are affecting elastic structures and are modified by the oblique PEL insertion, a striking, trumpet-like flaring of the GE junction results.
The mystery of sphincter release has been that, anatomically, there are no
radial muscle fibers to be found that, like the ciliary body, would dilate the
lower esophageal sphincter. The muscles of Juvara and Rouget to which Jutras(45)
attributes this function, have never convinced their critics. They are only
mentioned in this connection to be disparaged. They may have been some artifact
of the dissection process. In any event we can, should they exist, rule them
out of this role because of the wide variability in the relationship of the
sphincter to the hiatus.
Yet, we know from mechanics that a force need not act only in line with the
contracting muscle. Forces can undergo vector resolution into separate components.
In the case of the LM, one component of the contractile force is resolved in
such a way as to open the sphincter. In this resolution, the PEL plays the essential
role.
PEL anatomy
The PEL inserts on the distal esophagus at the sphincter. According to Zaino
et al.(46) some fibers insert above
and some below the sphincter. This confirms the descriptions by surgeons who
have been interested in investigating the hiatal area.(47),(48)
Bombeck et al.(49) examined a large
number of autopsy specimens using a method designed to demonstrate the details
of PEL insertion on the esophagus as they were interested in evaluating the
hypothesis that contraction of the diaphragm opened the sphincter.
These authors found that the PEL originates from the inferior margins of the
esophageal hiatus as a continuation of the endoabdominal fascia. It then divides
into 2 layers. In most individuals, the lower division is little more than a
layer of loose areolar tissue easily fractured with a finger. It may be absent
entirely. It inserts at or below the level of the ora serrata - 1.4 cm below
it on the average.
The other, more substantial layer, is invariably present, often receiving
an additional contribution of fibers from the endothoracic fascia. It inserts,
on the average, 3.35 cm. above the ora serrata. The insertion is not linear,
but occupies an appreciable longitudinal extent - up to 1 cm, although generally
less than that. In addition, ". . . . a diffuse fibroelastic network of fibers
passes from the main, membranous body of the ligament to the sphincter area
of the esophagus in all cases." The point of insertion was taken as the distance
from the ora serrata to the fibers obviously taking the load when the PEL was
stretched. These details are important because they show that the PEL is exactly
designed for its sphincter opening function.
Contraction of the muscle fibers of the sphincter only closes it more tightly. If it is to open at all, a bolus must force it or an external influence must pull it apart. This force must be applied radially and equally in all directions or the result would only be a lateral displacement of the esophagus.
Vector resolution and thePEL
In the case of the LES, this is accomplished with great finesse by employing
a longitudinal force, that of LM
contraction. It is the vector resolution of that force in all directions that
opens the sphincter. The other vector component merely stretches the PEL.(50)
The PEL is essential to this vector resolution of force. If it did not exist,
the force would simply pull the stomach through the hiatus. Because of the this
restraint, however, the force of LM contraction is resolved into radial vectors
that open the sphincter.
The normal mechanism of sphincter opening, therefore, has three elements: 1.)
The circular muscle of the sphincter is "turned off" reflexly and 2.) The sphincter
is mechanically dilated by vector resolution in the horizontal plane of the
force of LM contraction. 3.) An advancing bolus has a wedge-like opening effect.
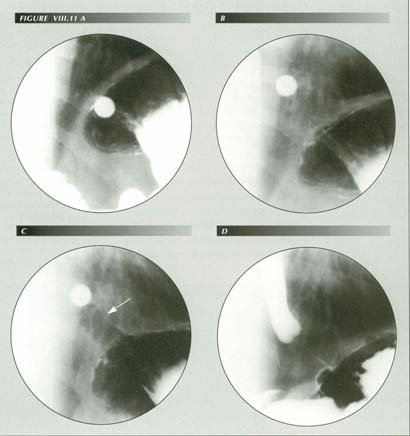
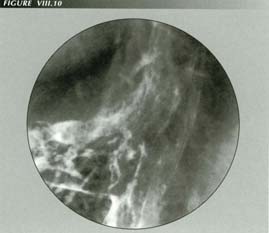
Figure VIII.11 A-D The esophagus “sees” foreign bodies. In (A) a barium tablet is arrested by a LER. Although it appears arrested below the diaphragm, it is merely below the dome. In (B) LM contraction has been provoked in an attempt to dislodge the tablet. Hiatal squeeze is still enough to prevent escape of gas from fundus. The diaphragm is becoming unsharp. At (C), the entire fundus and perihiatal region is elevated by the LM tension that has drawn a gas filled tube of stomach through the hiatus. The PEL tent surrounds this tube. (D) Later a Valsalva maneuver applies exernal pressure to the tube producing the “empty segment” appearance by inflating the PEL up to its insertion. It cannot be assumed that the introduction of foreign bodies such as endoscopes, pH meters, catheters, balloons and transducers will be physiologic. Here a small tablet has caused marked LMC and orad sphincter displacement. These and other effects such as sphincter release can invalidate manometric measurements or render them uninterpretable. The air filled gastric tube (arrow) could be mistaken for esophagus lined with gastric mucosa.
This precise integration of the functions of the longitudinal, circular and
sphincter muscles of the organ is necessary to carry out the esophageal function
in all positions and under a great variety of circumstances, some of which make
unusual demands.(52)
Non-peristaltic LMC
The LMC just discussed is but one mode of recognizable LM activity. The others
are en masse contractions rather than the progressive, relatively slowly
migrating contraction associated with peristalsis. They include:
Swallowing in the absence of resistance.
LM contraction during tertiary contractions.
Belching.
Gagging.
Vomiting.
In all these activities, the esophagus shortens and tents the PEL and even
the perihiatal region of the diaphragm itself. When the PEL is elongated to
any extent, it will assume the typical trumpet-bell shape of a membrane under
central tension. The retracted gastric fundus also becomes conical as it assumes
the same shape as the PEL tent. If the sphincter opens, it does so at the point
of maximal LM contraction.
The force of LM contraction can be gauged by the size of the conical tent; the higher and thinner the tent, the greater the traction being applied. There seems to be no basis for forming an opinion whether all of the muscle cells are partly contracted or some of the cells are completely contracted in this shortening. The speed of contraction seems to be greater the more forceful the contraction.
Swallowing liquids
During normal deglutition of barium in the upright position, LM contraction
is best seen if the patient is swallowing rapidly. As each bolus leaves the
esophagus, it does do on a slight up-stroke of the diaphragm. This
upstroke is easily mistaken for respiratory motion. However, it is detectable
because 1.) Respiration is suspended during deglutition and 2.) The rate of
diaphragmatic upstrokes is faster than the respiratory rate.
The upstroke is synchronized precisely with the spurt of barium from the esophagus
into the stomach. Thus, even with the assistance of gravity and with liquids
of low viscosity, LMC does come into play.
LMC and the manometric "plateau wave"
The upstroke of the diaphragm is caused either by an en masse contraction
of the LM, by the initial upward motion of the larynx to which it is attached
or, more likely, by both. Just as a sharp tap on its tendon may provoke a reflex
contraction of a skeletal muscle, the upward tug on the esophagus that initiates
swallowing may provoke a stretch reflex stimulating LMC.
Phase 1 of the manometer tracing - a negative pressure wave - is believed
to be due to stretching of the esophagus by the upward hyoid/laryngeal impulse.(53)
Phase 2 - a positive, plateau type wave - may well be a stretch reflex of the
LM.
As respiration is suspended during swallowing, this upward motion of the diaphragm about the hiatus translates into increased intrathoracic pressure. This raises the internal pressure of the esophagus throughout the organ so that manometers at multiple levels record a simultaneous pressure increase. The simultaneous pressure increase at all levels, its positive sign and its relation to the peak caused by the peristaltic wave passing the same level all identify this pressure increase as the "plateau" or "phase 2" portion of the deglutition wave.
In this indirect fashion the LM is able to affect a manometer. It may be the
single exception to my earlier statement that the LM is invisible to the manometer.
LMC in belching
The LM contraction that precedes belching is not an all or none evnt. The PEL
tent may be observed rising and falling for several seconds as escape of gas
from the fundus approaches. The incipient belch may be suppressed entirely,
in which case the tent is lowered and vanishes. If air does erupt, however,
it does so at maximal elevation of the tent, i.e., when LM contraction is sufficient
to open the sphincter. This is at once the most clear-cut and easily reproducible
way of demonstrating both the existence of a sphincter and that LM contraction
opens it. These phenomona are discussed in more detail in the chapter on gas/bloat.
Examination of the cine footage made available to me by Dr. William Dougherty showed that there is also LMC preparatory to eructation of gas by sheep. As the rumen is inflated with gas via a rumen fistula, repeated, forceful LM contractions occur as the animal attempts to belch. This was most striking in the cervical esophagus - a phenomenon Dougherty referred to as "fluttering." The activity slows or stops after a belch and resumes on reinflation of the rumen.
LMC in gagging
A type of contraction perceived by patients - and examiners for that matter
- as a gag also has esophageal manifestations. We are familiar with spasm of
the pharynx when the gag reflex is elicited, but this is only the oral aspect.
Abrupt LM contraction is the esophageal component of a gag. Although I subsequently
verified this effect repeatedly by having an assistant induce a gag with a tongue-blade
while I watched the cardia, I first became aware of this while examining the
following patient.
J.O. 6541 age 43. This man had a long history of "indigestion," "acid stomach,"
nocturnal acid regurgitation, pyrosis. "Food gets caught in that tube." and
he can't swallow. He regurgitates unchanged food.
Fluoroscopic note: The patient swallowed barium with great difficulty due to
a hypersensitive gag reflex. He was, nevertheless, very cooperative and swallowed
despite the difficulties, thus providing an extraordinary opportunity to view
the act of gagging. This proved to be an instantaneous longitudinal contraction
of the esophagus, jerking all the landmarks cephalad. The amplitude of the motion
was considerable because a rupture of the phrenoesophageal attachments allowed
gross hiatal herniation of the untethered fundus when the esophagus contracted.
LMC in nausea
Whereas tonic LMC during belching has an appearance of delicate control -
power applied with finesse - the LM contraction seen in nausea is a much more
powerful application of force to the lower esophageal attachments. In a fraction
of a second, an abrupt jerk elevates the PEL tent. It may not release the sphincter
even though the elevation is obviously more powerful than the force necessary
to release a belch. The contraction may partially subside before again increasing.
Because it is precisely at this preliminary stage of nausea and vomiting that
hypersalivation occurs, it suggests that stretching the PEL is the stimulus
to the hypersalivation that precedes vomiting. This has been observed in studies
of farm animals. Reid and Phillipson(54) showed
that distention of the rumen provoked increased salivary secretion. Clark and
Weiss(55) reported reflex salivation in sheep
and goats when an area about the cardia was stimulated mechanically as did Comline
and Kay.(56)
The LMC component of nausea, therefore, provides an explanation of the familiar hypersalivation that precedes vomiting. A powerful LMC applies traction to the PEL, stretching it beyond the limits normal for belching. This reflexly stimulates salivation by traction on the cardia. This stretching is perceived as nausea. Because of the alkaline pH of saliva, the hypersalivation has the effect of immediately neutralizing the acid pH of the esophagus after emesis.
The patient studied by Shay et al.(57) tends to confirm the mechanism. He had copious salivation caused by singultus. As is noted elsewhere, hiccups have the same mechanical effect on the cardia as LMC.
Vomiting
Finally, the most severe degree of LM is perceived as the pain and gagging
sensations of vomiting. Because the sphincter must be open before the stomach
can be evacuated, emesis calls forth a powerful LMC that rides roughshod over
sphincter resistance. There is nothing subtle or tentative about this form of
LM contraction. The stomach is yanked into the hiatus to the full extent of
the PEL's ability to stretch by a powerful, almost instantaneous LM contraction
as the stomach contents are discharged.
To observe the process fluoroscopically the radiologist's instinct for keeping
barium out of his shoes must be overcome. Generally one steps back and shouts
for the emesis basin at the first sign of gagging and spasmodic abdominal muscle
contraction. Perhaps this is the reason standard references fail to mention
the LM in connection with vomiting.
This powerful contraction is the reason Daintree Johnson(58)
was able to produce HHs in dogs by inducing vomiting with apomorphine.(59)
Forceful LM contraction is also the reason patients note subxiphoid
soreness for some time after emesis. It is a painful contraction, because it
over-stretches the PEL. The discomfort is a part of the reason we struggle to
avoid vomiting. Infants trying to burp often scream from the pain of LM contraction
stretching the esophageal attachments to the diaphragm in an attempt to open
the sphincter.(60)
From time to time reports of retrograde prolapse of the gastric mucosa or
gastroesophageal intussusception are encountered.(61)
These can generally be shown to be examples of retching or variants of the captive
bolus phenomenon. The LMC draws the stomach through the hiatus as wire is drawn
through a die. Through an endoscope the gastric mucosa may be seen reaching
8-10 cm above the gastroesophageal junction.(62)
Again, the salient feature of contraction of the LM is that in every instance in which it is seen - belching, nausea, vomiting, cardio-esophageal reflux - the sphincter is effaced.
LMC causes both Mallory-Weiss and Boerhaave syndromes
Retching, an act indistinguishable from gagging, is another manifestation
of LMC. The LMC may not succeed in emptying the stomach on its first attempt
or the stomach may be empty. The contractions are the same and are accompanied
by nausea and hypersalivation. Retching is essentially an aborted emesis.
A cause of about 10% of upper gastrointestinal bleeding, the Mallory-Weiss
syndrome, starts with retching or non-bloody vomiting followed by hematemesis.
This pattern has always suggested that the initial retching itself caused the
bleeding. About 10% of the cases are due to retching during endoscopy (63),(64)
providing ample opportunity to confirm the etiology as the endoscopist observes
intact mucosa on inserting the instrument, then retching, and subsequently sees
the linear tear(s) as he withdraws it.
Knauer(65) observed 58 cases noting that 72%
had HHs. There was a striking radial asymmetry in the location of the tears
with 52% occurring on the right vs. only 7% anteriorly. In other series,(66)
the incidence of HH has been as high as 100%. The friability of the gastric
mucosa within the "hernia" is cited as a factor in the ease of mucosal rupture.
LM tension causes vomiting: Here the patient is trying to vomit. He habitually induces vomiting to relieve the sensation of left upper abdominal pressure Severe heartburn. Acid regurgitation bujt no cheilosis. Frequently a wet spot on pillow in AM. Numerous dental caries, frequent sore tongue. Mild duodenitis. (A) Severe esophagitis: There are no signs of HH with the LM relaxed. (B) The remarkable force of LMC has stretched the PEL as the patient retches. Spontaneous mass contraction drew the fundus 8.5 cm aboe the diaphragm. This happened slowly enough over a period of about 10 seconds that the patient could be askede whether he was having the “pressure” sensation that was his chief complaint. He emphatically responded that he was. Questioned a few seconds later, after the LMC had subsided, he reported that the “pressure” sensation was gone. In vomiting this sequnce occurs almost instantly. The LM has contracted 37% of its length(8. cm/23 cm). One can imagine what this would do to reconstructive surgery about the hiatus
Like effacement of the sphincter, these syndromes present the paradox of a
longitudinal force producing, not the expected transverse tear, but a longitudinal
one. Although they are attributed to overdistention of the esophagus or herniated
cardia by sudden ejection of gastric contents, this can scarcely be the case
as they are seen after retching without emesis and after endoscopy that, of
course, is performed on an empty stomach. The wedge shape of the tears(67)
observed after endoscopy-induced retching is a clue that the force is applied
at the PEL. If overdistention caused them, they would tend to be elliptical.
It is, perhaps, puzzling that most of the tears (78%) are in the stomach just
below the mucosal junction. Two circumstances may account for this.
1.) 72% to 100% [Knauer] of the patients have hiatus hernias. The increased
friability of the mucosa in the herniated portion of the stomach may account
for this localization.
2.) LMC, when resolved by the PEL, causes a trumpet-like flaring of the GE junction. The further down the trumpet, the more the mucosa is stretched. This accounts for the endoscopic observations that wide end of the wedge-shaped tear is aboral and that virtually all of the tears are below the ora serrata.
LMC in myotonia dystrophica and scleroderma
The esophagus of myotonic dystrophy provides an elegant confirmation of the
proposition that the LM opens the sphincter. In this disease, characterized
by a deficiency in the ability of muscle to relax, if the LM is affected, it
may be constantly contracted. For that reason, the p-wave cannot latch the sphincter.
Constant LMC keeps the sphincter constantly open. This results in an appearance
that, like scleroderma, can be mistaken for achalasia because of the striking
air esophagram it produces.
This identifies another important sphincter function: it keeps gas out of
the esophagus as well as releasing it from the stomach. If the stomach and esophagus
are in constant communication, the circular muscle cannot collapse the lumen.
No matter how often a peristaltic wave milks gas into the stomach, if the sphincter
does not latch, air rushes back to again distend the body of the esophagus.
But distention is the stimulus to peristalsis ("The esophagus abhors distention."
[Dodds]), so the process repeats to the exhaustion of the circular muscle.
When these patients are upright, a continuous air column extends from the
superior constrictor of the esophagus through a widely patent sphincter nearly
to the gastric antrum. The only way the patient can prevent reflux in the standing
position is to swallow so much air that the fluid level never reaches the esophagus.
As in achalasia, the circular muscle can never rest and the esophagus ends as
a dilated, aperistaltic tube.
The situation in scleroderma is similar. The LM is constantly short - nearly
all such patients have tubular HHs, many of which are unrecognized in the published
cases. The mechanism is not clear, but these patients end up with atrophy
of the CM while the LM is preserved.
Longitonia
Belching, retching, gagging and vomiting, are isolated events that occur to everyone. We also have to deal with a pathologic state of the LM in which its tone or irritability are increased. In its purest form, LMC causes a symptom complex for which esophageal longitonia might be an approprate nane. The salient symptom of this abnormality, reflux, requires a separate chapter.
Clonic LM contraction and pseudo-palpitations
There may also be a clonic type of LM contraction that passes for "cardiac
palpitation." From personal experience over many years, I can describe the sensation
produced as a jerking, thumping, palpitating sensation in the mid substernal
region. These palpitations are so irregular that they remind one of the erratic
thrashings of a recently caught fish flopping about on the bank of the stream.
So alarming were these "palpitations" that, as a pre-medical student I once
called the Student Health Service for assistance.
Although I had never been able to palpate a pulse irregularity, I was satisfied
with a medical opinion I was having premature ventricular contractions until
I experienced such an episode while I was in the radiology department of a small
hospital. The EKG room was next door and the same nun was both x-ray and EKG
technician, so, to clear up the mystery, I was able to schedule an EKG instantly.
As I watched the tracing emerge from the machine, I was totally unprepared
for the normal rhythm it charted. Ruminating on this over the years, by a process
of elimination, I formed the suspicion that the sensation could be due to clonic
contraction of the LM. If it were not the heart, barring the internal thoracic
muscle, the only other muscular organ in the vicinity was the esophagus.
I then recalled that patients with auricular fibrillation and a totally irregular
heart beat seldom complained of palpitation. With this in mind, "Have you ever
had palpitations or a sensation like a fish flopping around in your chest?"
became a part of my routine questionnaire.
Surprisingly, many patients with esophageal disorders did have palpitations.
Among them were several physicians. The latter were curious enough, as I had
been, to check their pulses for the compensatory pause of this common arrhythmia.
Like me, some failed to detect it, but tended to attribute that to the difficulty
of reading one's own pulse. Actually, anyone with some medical training who
has had PVCs has no difficulty detecting the irregularity in the radial pulse.
Convincing proof of my hypothesis was eventually forthcoming from an unexpected
source. A patient was seen for a followup examination a year or so after a "pull-down"
operation for hiatus hernia. In this, the original Nissen operation, the lesser
curvature of the stomach is sutured to the posterior surface of the left rectus
sheath. To my routine question she responded that she had had cardiac palpations
before her operation. Did she still have them? "No," she said, (pointing to
the left rectus area), "but now I get this tugging sensation in my abdomen."
It is worth noting that each mode of LM contraction is perceived by the patient as a distinctly different sensation. LM contraction is the basis for explaining a number of very common but misunderstood symptoms.
Nausea
A more severe degree of tonic LM contraction, like hypersalivation, is perceived
as a part of the nausea syndrome. It would be interest to determine if ataractic
drugs act by decreasing LM tone and irritability. The difference between nausea,
"gas" and reflux-inducing LM tone is one of degree. The relation between LM
contraction and the sphincter will be discussed in detail when gastro-esophageal
reflux is described.
Substernal pain and the LM
With substernal pain, the main effort is to distinguish between esophageal
pain and cardiac ischemia. Bennett and Atkinson(68)
tabulated the details of location, radiation, relation to effort and posture,
aggravating and ameliorating influences in 200 consecutive admissions for precordial
pain. A variable overlap of every symptom nuance was found with sizable variations
in the percentages for ischemic heart disease and esophagitis - enough to suggest
that a Bayesian analysis might be a practical (but expensive) means of differentiating
the two. One of the more accurate predictors was simply asking the patient whether
he though he had heart trouble or indigestion. The patient's diagnosis was correct
in 61% of the cases with ischemic heart disease and 85% of the cases with indigestion
- a distinct improvement on the accuracy of the admission diagnosis of 64% overall.
In contrast to the generally clear-cut radiologic and EKG findings in chest
pain of cardiac origin, the correlation of symptoms with objective manometric
findings in non-cardiac chest pain is very poor. Patients may show no increase
in intraesophageal pressure at all during an attack.
Clause et al.(69) carefully selected
patients with daily substernal pain unrelated to exertion and without any identifiable
cause other than presumed esophageal spasms. They compared the manometric recordings
of 9 such patients with control periods in which no pain was present. The tracings
were studied blind so that the interpreter was unaware of the presence or absence
of pain.
There were no significant differences between recordings during or preceding
an episode of pain and those made in pain-free periods. There were no significant
differences in baseline esophageal pressure or peak pressures. The amplitude,
duration and percent of abnormal peristalsis were well within the limits established
in the control periods. In 3 subjects, no waves at all were seen during pain
periods. Thus, despite the widespread suspicion that substernal pain is esophageal
in origin, none of the patients had a recorded change in usual motility pattern
that correlated with the occurrence of the reported pain episodes. They concluded
that abnormal contractions are not the direct cause of pain.
Cold food can also be a cause of esophageal pain, but ice cream induced esophageal
pain results in aperistalsis(70) -
another result inexplicable in terms of abnormal sphincter or peristaltic activity.
Clause et al. suggested that perhaps these peristaltic abnormalities
may be markers for some unrecognized cause of chest pain because motility aberrations
are so common in patients with substernal pain and no apparent cardiac disease.
Clinical manometry may demonstrate intraluminal pressure of 300 mm Hg or more
in patients with pain of esophageal origin, but there may be no pain at the
time the high pressures are being recorded. Ott et al.(71)
reported a high incidence of tertiary activity in their series of 20 cases labeled
nutcracker esophagus(72) but
stated that the significance of the finding was unknown. The incidence of HH
(70%), reflux (15%), diverticula (15%) and TCs (50%) was dismissed as nonspecific
incidental findings.(73) In a typical
study,(74) 24-hour ambulatory pH/motility
monitoring showed that only 21% of chest pain episodes correlated with motility
abnormalities.
Because of these discrepancies, it is reasonable to suspect that the spasm causing the pain is not in the CM or sphincter, but in the longitudinal muscle as this will escape detection in the studies listed. There are several clues:
1. LMC applies traction to the diaphragm and so can produce pain.
2. Vomiting, which entails a severe contraction of the LM, is painful.
3. In DES, there is powerful LMC as well as increased peristaltic activity.
4. The pain may disappear after rupture of the phrenoesophageal attachments.
A patient, age 43, gave a history of left subchondral pain diagnosed as nervous
stomach while in the army 7 years previously. It was associated with heartburn
and "doubled me up with pain" persisting 30-90 minutes before subsiding. Eventually,
although the heartburn persisted, the painful attacks ceased as suddenly as
they had begun. On examination, he was found to have a 9 cm HH with rupture
of the PEL.
A possible explanation is that rupture of the PEL terminates the ability of
the LM to apply painful traction to the diaphragm. This would be consistent
with the finding of Dalton et al. that the natural history of nutcracker esophagus
is one of spontaneous remission.(75)
Radiologic followup of a number of such cases should prove interesting. A radiologic
search for signs of LM tension during a typical attack should be even more revealing.
There is no doubt that sub-xiphoid (wishbone) pain and/or tenderness is the
most common localization of discomfort in patients referred for an examination
of the upper GI tract. For many years I have asked each upper GI patient to
identify the site of pain by pointing to it. For at least 10 of these years,
I was mystified that in most cases I could find no radiological explanation
for the symptom. It is not the site one thinks of as either duodenal or gastric
reference and may be present in the absence of pyrosis.
Eventually, it became clear that this is but one of the manifestations of LMC. Although frequently associated with pyrosis, it is not due to pyrosis per se, but to the stretching or tensioning of the esophageal attachment to the diaphragm. Less frequent are other references of diaphragmatic pain - subscapular, left arm and to the neck or even the ear. Anterior flexion of the cervical spine, which lessens esophageal tension may provide some relief.
SUMMARY
The longitudinal muscle of the esophagus plays a dominant role in most functions
of the organ. Its most important function is opening the LES. In peristalsis
it undergoes a latching type of contraction. It also exhibits several nonperistaltic
modes of contraction. These are most commonly the en masse contractions
that are associated with nausea, vomiting and belching.
Severe tonic contraction is perceived as subxiphoid pain or as nausea. An abrupt
en masse LM contraction of marked degree is a gag. The most severe
en masse contractions cause nausea and vomiting. The mechanical stimulation
of the cardiac region by LMC causes hypersalivation. LM contraction alone without
associated diffuse spasm or nutcracker contractions can produce severe pain.
Clonic LM contraction may well be the cause of sensations perceived as cardiac palpitation.
References
Last Updated July 27, 2007 by WRS Press
1. 1. Esophageal Function in Health and Disease, Eds. Castell, Donald O., Johnson, Lawrence F., Elsevier Biomedica, New York, 1983.
2. 2. Bockus Gastroenterology, Fourth Edition, Vol 2, Ed. Berk, J. Edward, W.B.Saunders Company, Philadelphia, 1985.
3. 3. Ziano, Constantino and Beneventano, Thomas C., Radiologic examination of the oropharynx and esophagus, Springer-Verlag, New York, 1977.
4. 4. Esophageal function in health and disease. Eds Castell, Donald O. and Johnson, Lawrence F, Elsevier Biomedical New York, 1983.
5. 5. Diamant, Nicholas E., Esophageal physiology. In: Diseases of the esophagus. Eds. Cohen, Sidney and Soloway, Roger D., Churchill Livingstone, New York, 1982.
6. 6. Earlam, Richard, Clinical tests of oesophageal function, Grune & Stratton, New York, 1975.
7. 7. Hurwitz, Alfred A., Duranceau, Andre, and Haddad, Jean K., Disorders of Esophageal Motility, W.B. Saunders Co, Philadelphia, 1979.
8. 8. Gentile, R., et al. Comparative anatomo-microscopic observations on the esophagus of teleosts with different feeding habits. Bollettino - Societa Italina Biologia Sperimentala 69:137-44, 1993.
9. 9. Pelot, Daniel, Applied anatomy and anomalies of the esophagus. In: Bockus Gastroenterology, Fourth Edition, Vol. 2, Ed. Berk, J. Edward, W.B. Saunders Company, Philadelphia, 1985.
10. 10. Netter, Frank H. and Mitchell, G.A.G Anatomy of the Esophagus in The Ciba Collection of Medical Illustrations, Volume 3, Part 1, Ciba Pharmaceutical Products, Inc., New York, 1959.
11. 11. Meyer, G.W and Castell, D.O., Anatomy and physiology of the esophageal body, in Esophageal function in health and disease, Eds. Castell, D.O. and Johnson, L.F., Elsevier Biomedical, New York, 1983.
12. 12. Meyer, G.W. and Castell, Anatomy and physiology of the esophageal body. In: Esophageal function in health and disease. Eds Castell, Donald O. and Johnson, Lawrence F, Elsevier Biomedical New York, 1983.
13. 13. Kaufman, P., Lierse, W., Stark, K and Seltzner, F., Die muskelanordnung in der spiesrohre. Ergbn. Anat. Entwickl-Gesch. 40: Heft 3, 1968.
14. 14. Diamant, Nicholas E., op cit..
15. 15. Christensen, James and Lund, Gordon, Esophageal response to distention and elect rtical stimulation. J. Clin. Invest. 48: 408-19, 19
16. 16. In: Bockus Gastroenterology, Fourth Edition, Vol. 2, Ed. Berk, J. Edward, W.B. Saunders Company, Philadelphia, 1985.
17. 17. Netter, Frank H. and Mitchell, G.A.G Anatomy of the Esophagus in The Ciba Collection of Medical Illustrations, Volume 3, Part 1, Ciba Pharmaceutical Products, Inc., New York, 1959.
18. 18. Srivasta, A.K., Vanaqunas, A., Kamel, P and Cooper, R., Endoscopic ultrasound in the evaluation of Barrett's esophagus: a preliminary report. Am. J. Gastroenterology 89:2192-5, 1994.
19. 19. Earlam, Richard, op cit.
20. 20. Rywlin, Arkadi M. and Ortaga, Rolando, Glycogenic acanthosis of the esophagus. Arch. Path. 89-90: 439-43, 1979.
21. 21. Hayward, J., The lower end of the esophagus. Thorax 16: 36-41, 1961.
22. 22. Groszek, Irena and Matysiak, Wlodzimierz, Morphologie der phreno-esophagealen Membran, Gegenbaurs morph Jahrb. 131: 1-18, 1985.
23. 23. Christensen, James and Lund, Gordon F., Esophageal response to distention and electrical stimulation. J. Clin. Invest. 48: 408-19, 1969.
24. 24. Sugarbaker, D.J., Rattan, S. and Goyal, R.K. Mechanical and electrical activity of esophageal smooth muscle during peristalsis. Am. J. Physiol. 247: 515-9, 1985.
25. 25. Janssens, J., The peristaltic mechanism of the esophagus. Acco, Leuven, 1978. cit.ed by Diamant In: Diseases of the esophagus. Eds. Cohen, Sidney and Soloway, Roger D., Churchill Livingstone, New York, 1982.
26. 26. Kerlin, Paul, McCafferty, Gerald J., Robinson, David W. and Theile, David., Function of a free jejunal conduit graft in the cervical esophagus. Gastroenterology 90: 1956-63, 1986.
27. 27. Barone,F.C., Lombardi, D.M. and Ormsbee, H.S. III, Hindbrain control of lower esophageal sphincter in the cat. (Abstract) Gastroenterology 84: 1098, 1983.
28. 28. Christensen, James and Lund, Gordon F., Esophageal response to distention and electrical stimulation. J. Clin. Invest. 48: 408-19, 1969.
29. In Boerhaave's famous case it avulsed the esophagus from the stomach.
30. 29. Hatfield, Malcolm and Shapir, Jonathan, The radiologic manifestations of failed antireflux operations, AJR 144: 1209-14, 1985.
31. 30. Burhenne, Linda J. W., Fratkin, Leonard B., Flak, Borys, et al., Radiology of the Angelchik prosthesis for gastroesophageal reflux. AJR 142: 407-11, 1984.
32. 31. Lewis, R.A, Angelchik, J., and Cohn, R., A new surgical prosthesis for hiatus hernia repair. Radiology 135:630, 1980.
33. 32. Lackey, C., and Potts, J., Penetration into the stomach - a complication of the anti-reflux prosthesis. JAMA 248: 350, 1982.
34. 33. Stillwell, G. Kieth, The law of Laplace. Proc. Mayo Clin. 48: 863-9, 1973.
35. 34. Torrance, H. Bruce, Studies on the mechanism of gastro-esophageal regurgitation. J. Roy. Co. Surg. (of Edinberg) 4: 54-62, 1957.
36. Torrance does not mention the effect of such shortening on the PEL, however,I have seen shortening of nearly 50% in a vomiting infant without any evidence of a tear or even permanent stretching of the PEL.
37. Two thirds of the feline esophagus is striated muscle.
38. 35. Edwards, M.H., Selective vagotomy of the canine oesophagus - a model for the treatment of hiatal hernia. Thorax 31: 185-9, 1976.
39. 36. Matthews, H.R., Thorpe, J.A.C. and Little, G., Effect of vagal stimulation on the normal and abnormal esophagus. In: Esophageal Disorders, Eds. DeMeester, Tom R. and Skinner, David B., Raven Press, New York, 1985.
40. Judging from animal experiments, it may be the other way around. The integration of LM and CM contraction is more complex than suggested by the fluoroscopic appearance. This is really the resultant of LMC and LM relaxation both of which may be taking place at once. Using miniature strain gages to measure LM and CM contraction, Sugarbaker, et al. found that in laboratory preparations both LM and CM contracted sequentially during peristalsis but the propagation was faster in the LM than in the CM and the contraction lasted longer. The duration of contraction was
also longer in the distal than in the proximal esophagus.
41. 37. Sugarbaker, D.J., Rattan, S., Goyal, R.K., et al., Evidence for central sequential activation of esophageal longitudinal muscle during swallow induced peristalsis. (Abstract) Gastroenterology 86: 1270, 1984.
42. 29.38. Dodds, Wiley, et al., op cit.
43. 39. You, Chul H., Lee, K.Y., Chey, William Y., et al., Electrogastrographic study of patients with unexplained nausea, bloating and vomiting. Gastroenterology 79: 311-4, 1980.
44. 40. Christensen, James and Lund, Gordon F., op cit..
45. 41. Jutras, A., Levrier, P. and Longtin, M., Etude radiologique de l'esophage paradiaphragmatique. J. de Radiol. et Elect. 30: 373-416, 1949.
46. 42. Ziano, C., Poppel, M.H., Jacobson, H.G., Lepow, H., and Osturk, C.H., Lower esophageal vestibular complex: anatomic Roentgen study. Am. J. Roentgenol., Rad. Therapy & Nuclear Med., 84: 1045-1055, 1960.
47. 43. Allison, P.R, Reflux esophagitis, sliding hiatal hernia, and the anatomy of repair. SG&O *** 419-31, 1952.
48. 44. Hayward, J., Phreno-esophageal ligament in hiatus hernia repair. Thorax 16: 41-45, 1961.
49. 45. Bombeck, C. Thomas, Dillard, David H. and Nyhus, Lloyd M., Muscular anatomy of the gastroesophageal junction and the role of phrenoesophageal ligament: autopsy study of sphincter mechanism. Ann. Surg. 164: 643-54, 1966.
50. Although it seems paradoxical that a force applied longitudinally has a transverse effect, there is no question of its effectiveness. The same force resolved in the same way can tear the mucosa (Mallory-Weiss syndrome) or rupture the wall (Boerhaave's syndrome) and in each case, the tears are almost always longitudinal. See appendix B.
51. Pro-Engineer, a product of Parametric Technology Corp.
52. Some years ago, the Milwaukee Journal published
a Metro Group photograph of a group of teammates doing handstands over the following
caption:
"Lucki Hofmaier was so good at doing handstands and drinking beer that he decided to form a team in Regensburg, West Germany, to encourage competition between other West German cities. Hofmaier can suck a glassful of beer through a straw in about 5 seconds while doing a handstand."
53. 46. Ingelfinger, Franz J., Esophageal motility. Phys. Rev 38: 533-84, 1958.
54. 47. Reid, C.S.W and Phillipson, A.T., Distention of the rumen and salivary secretion. Nature 181: 1722-3, 1958.
55. 48. Clark, R and Weiss, K.E., Reflex salivation in sheep and goats initiated by mechanical stimulation of the cardiac area of the forestomachs, South African vet. Med. Ass. 23: 163-5, 1952.
56. 49. Comline, R.S., and Kay, R.N.B., Reflex secretion of the parotid gland of the sheep. J. Phsiol. 129: 155, 1955.
57. 50. Shay, Steven DS., Myers, Robert L. and Johnson, Lawrence F., Hiccups Associated with reflux esophagitis. Gastroenterology 87: 204-7, 1984.
58. 51. Johnson, H. Daintree, op cit.
59. In this connection, Johnson unearthed interesting supportive anatomical evidence: rats, which cannot vomit, have a very thin LM - only a few cells thick. Cats, on the other hand, vomit with great facility and have a thick LM.
60. These two examples of LMC induced pain add weight to the presumption that noncardiac chest pain has a similar cause.
61. 52. The Captive Bolus Test and the Pinchcock at the diaphragm, AJR 99: 223-32, January 1967.
62. 53. Buhac, Ivo, Gastric mucosal prolapse or gastroesophageal intussusception. Dig. Dis. Sci. 30: 190, 1985.
63. 54. G.C. and Kahwaja, F.I., Iatrogenic Mallory-Weiss tears: incidence and associated findings. (Abstract) Gastrointest. Endoscopy 31: 164, 1985.
64. 55. Holmes, G.K.T., Mallory-Weiss syndrome., Lancet 2: 161, 1978.
65. 56. Knauer, C. Michael, Characterization of 75 Mallory-Weiss lacerations in 528 patients with upper gastrointestinal hemorrhage. Gastroenterology 71: 5-8, 1976.
66. 57. Dagradi, Angelo E., Broderick, Joseph T., Juler, George, et al., The Mallory-Weiss syndrome and lesion. Am. J. Dig. Dis. 11: 710-21, 1966.
67. 58. Baker, Robert W., Spiro, Alan H. and Trnka, Yvona M., Mallory-Weiss tear complicating upper endoscopy: Case reports and review of the literature. Gastroenterology 82: 140-2, 1982.
68. 59. Bennett, John R. and Atkinson, Michael, The differentiation between esophageal and cardiac pain. Lancet 3: 1123-7, 1966.
69. 60. Clouse, Ray E., Staiano, Anamariea, Landau, David W. and Schlachter, Jeffrey L., Manometric findings during spontaneous chest pain in patients with presumed esophageal spasms. Gastroenterology 85: 395-402, 1982.
70. 61. Meyer, G and Castell, D.O., Human esophageal response during chest pain induced by swallowing cold liquids. JAMA 246: 2057-9, 1981.
71. 62. Ott, David J., Richter, Joel E., Wu, Wallace C., Chen, Yu Men, Gelfand, David W. and Castell, Donald O., Radiologic and manometric correlation in nutcracker esophagus. AJR 147: 693-5, 1986.
72. Evidently because of the high pressure - 350 mm Hg or more - recorded on manometry.
73. We have seen, however, that these findings are all manifestations of LMC.
74. 63. Peters, L.J., Maas, L.C., Dalton, C.B., et al., Spontaneous non-cardiac chest pain. Evaluation by 24 hour ambulatory esophageal and pH monitoring. Gastroenterology 94:878-86, 1988.
75. 64. Dalton, C.B., Bradley, L.A., Castell, D.O., et al., The nutcracker esophagus. Long-term clinical followup. (Abstract) Gastroenterology 91: 1050, 1986.