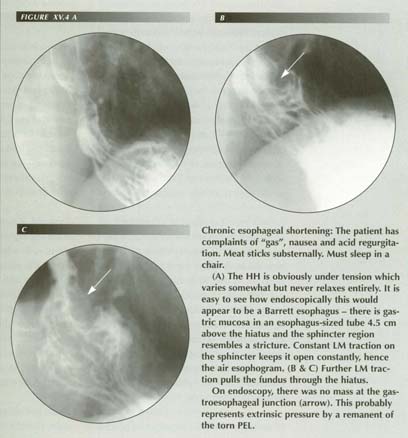
The (dubious) Barrett esophagus
"Barrett esophagus" (BE), an esophagus lined with gastric mucosa, is a popular
endoscopic diagnosis. Its incidence appears to be increasing rapidly as its
lore proliferates. Both Winters et al.(1) and
Schnell, et al.(2) have reported a 12.4%
incidence of BE among adult patients with gastroesophageal reflux - a remarkable
epidemic for a disease that may be non-existent. A reviewer(3)
suggests that radiologists should also be learning to make the diagnosis.
Some will recall that the literature of the 1940s contained many reports of
"congenitally short esophagus with intrathoracic stomach," an entity that vanished
after it was pointed out that to prove such a diagnosis it would be necessary
to show that the blood supply to a thoracic stomach originated in the thorax.
Perhaps it is not coincidental that the advent of the Barrett esophagus (1950)
was simultaneous with the demise of the "congenitally short esophagus." The
two may be opposite sides of the same coin - complementary ways of misdiagnosing
hiatus hernia. Proof that a tube of gut lined with gastric mucosa is esophagus
would require demonstration that it is supplied by esophageal, not gastric,
blood vessels. Thus far, I know of no case in which this criterion is satisfied.
The existence of BE is solidly based on assertion. As Levine states, ". .
. it was postulated . . . ". It is hard to account for the extraordinary attractiveness
of this conjecture. It seems to have gained universal, if uncritical, acceptance
with the lone exception (albeit temporary) of Barrett himself.
Radiologists seldom make the diagnosis of BE (all they see is a tubular hiatus
hernia).(4) Endoscopy, although presumed to be
the gold standard in the diagnosis of BE, is a fallible method. The presence
of gastric mucosa closer to the incisors than normal does not establish the
diagnosis. It merely proves that the esophagus is shorter than normal, as it
is in many hiatus hernias. Experimental esophagitis by acid perfusion causes
esophageal shortening in the opossum.(5),(6)There
is evidence that the same is true in human esophagitis,(7)
a condition that is invariably present when BE is reported.
To establish that he is biopsying esophageal mucosa, the endoscopist must
first determine internally where the esophagogastric junction is located. There
are two landmarks: 1.) The sphincter and 2.) The squamo-columnar junction. The
first, however is a manometric(8), not an endoscopic
landmark(9) and the second is what he must postulate
is misplaced. Spechler and Goyal, who have written extensively on the subject,
state, ". . . one cannot determine with certainty where the esophagus ends and
the stomach begins." If this is the case, how can an endoscopic diagnosis of
BE be made? In practice, there is little doubt that the diagnosis is based on
the distance from the incisors at which gastric mucosa is encountered.
In the Veterans Affairs Cooperative Study of BE(10),
20% of 93 patients had the diagnosis on only one of two examinations made six
weeks apart. The endoscopically determined LES was 3 cm proximal to that determined
by manometry. One criterion for diagnosis was the presence of 3 or more cm of
specialized columnar epithelium above the manometrically determined LES. The
other was biopsy of "specialized columnar epithelium" (SCE) in "tubular esophagus."
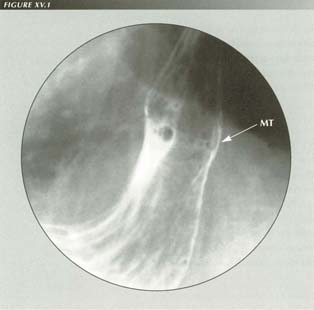
LMC with tubular HH: The short esophagus with a tubular HH can easily be mistaken for an esophagus lined with gastric mucosa. Note the mucosal transition (MT) and compare with published cases of BE. Distension of the esophagus with air causes reflex LMC producing this appearance.
The outliers in these statistics are most significant. On a second examination, the most proximal level of Barret's epithelium changed from as much as 7 cm lower to 8 cm higher in patients who had not had surgery in the interim. Kim et al. concluded ". . . approximately 10% of patients had a change 4 cm on endoscopy and manometry between examinations. This led to an apparent change in diagnosis in 18% of patients with Barrett's esophagus."
It is clearly impossible that 4 cm or more of "metaplastic" gastric mucosa
could revert to squamous mucosa in 6 weeks; on the other hand, it is certain
that the amount of stomach above the diaphragm will vary, not only from one
examination to the next but from moment to moment in the same examination. Greater
or lesser inflation of the esophagus will produce more or less LMC. Gastric
mucosa above the hiatus is a hiatal transtract - not metaplastic squamous epithelium.
The BE population is a subset of the esophagitis-GER population. Statistically,
it could be expected that most of the patients in the VA study would have hiatus
hernias. As LM tension both opens the sphincter (producing reflux) and stretches
the PEL (producing hiatal transtraction), the two are inseparable. Remarkably,
none of the 116 patients identified as having both severe GER disease and BE
were reported to have HHs!
A Medline search of the 1990 to date database yielded 205 abstracts for the
keywords ("hernia" and ("hiatus" or 'hiatal")),
350 for "Barrett" and 11 for the intersection of the two. Of the 11 several
were miscodes. Several were not actual case reports, some were didactic. One
of the latter baldly stated that 75% of BEs had HHs. So we have here the same
Venn diagram as with achalasia-hiatus hernia, forcing the same conclusion: the
two do not occur together because they are the same thing - now diagnosed
one way, now the other.
Biopsy "proof" of BE is unconvincing for two reasons: 1.) The pathologist can only describe the mucosa. The muscular layers - although even these would not be unequivocal - are not included in the specimen. To make the diagnosis of BE from a biopsy pathologists must rely on supporting information from the endoscopist. That information is usually the distance below the incisors at which the biopsy was taken or the distance above the manometrically determined sphincter. The endoscopist becomes a self-fulfilling prophet. 2.) The pathologist must be pre- indoctrinated that intestinal metaplasia of gastric mucosa is metaplastic stratified squamous epithelium.
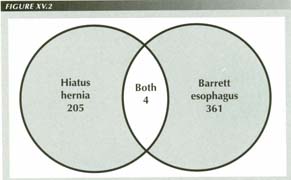
In the 1990 to date Medline database, although there were many articles on HH and on BE, there were only 11 in which the abstract mentioned both keywords. After deleting the miscodes, only 4 of this 11 mentioned patients with both disorders. As both the BE and the HH populations are subsets of the GE reflux population, one would expect that at least 70% of the BE articles or 253 would deal with patients who had HHs as well, not the 4 that actually did so. Equally remarkable, 97% of the articles on HH failed to mention BE. The conclusion is that HHs are being diagnosed BE and vice versa, or, more likely, they are the same thing.
Even at autopsy a pathologist would have difficulty determining whether a supradiaphragmatic
tube of gut lined with gastric mucosa is esophagus or a tubular hiatus hernia.
The blood supply is destroyed by the usual Rokatansky autopsy technique that
transects the viscera at the diaphragm before removal.
The unproven assumption on which BE rests rivals the audacity of the achalasia
assumption that a loss of motor neurons will cause a muscle to hypertrophy.
The postulated metaplasia from squamous to highly specialized columnar epithelium(13)
is a false analogy - backward in fact. Whether it is the lung, the cervix, the
endometrium, the gallbladder, the pancreas, the urinary tract or the bile ducts,
metaplasia replaces a specialized glandular, columnar epithelium with
less specialized epithelium. Usually this is stratified squamous epithelium
although gastric mucosa may convert to the less specialized intestinal mucosa
as indeed it does in cases claimed to be BE. I have been unable to find reports
of reverse metaplasia elsewhere in the GI tract or in any other organ.(14)
The burden of proof of BE, therefore, rests on those who postulate that, in
the esophagus, it is the other way about. One might expect to encounter islands
of gastric mucosa on the tongue or in the labial fissures if this were a possibility.
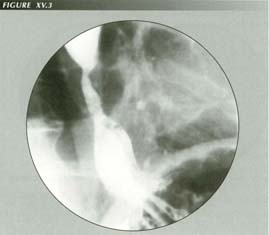
Barrett esophagus: The short esophagus + stricture + tubular HH shown here may appear to the endoscopist to be a Barrett esophagus. Acid reflux can cause esophageal shortening. If films are exposed for low contrast and low density, one can often see the external surfaces of these structures.
A transition from squamous to specialized gastric mucosa would be differentiation
in the technical sense. Tissues undergoing neoplastic transition - as it is
claimed to be true in BE - dedifferentiate. It is difficult to understand
how these contradictory concepts can be maintained in separate watertight compartments.
Biopsy of supposed cases of BE tends to refute the diagnosis. The histology
is also more in keeping with a tubular transtract than metaplasia. In addition
to the normal squamous lining of the organ, three types of mucosa are encountered.
Again, according to Spechler and Goyal, these occur in precisely the following
order from above to below:
A." Specialized mucosa." This is still recognizable as gastric mucosa but
distorted so as to be similar to intestinal metaplasia of gastric mucosa.
It is metaplastic, but metaplastic gastric, not esophageal, mucosal.
That is, the gastric mucosa is transformed in the usual way of metaplasia in
the direction of the less specialized intestinal mucosa.
B. "Junctional" mucosa. This is another name for the normal mucosa of the gastric
cardia.
C. "Fundic" This is the normal or somewhat atrophic mucosa of the gastric fundus.
Only the first of these would be considered abnormal. The three mucosal types
could be found consistently in some other order or in random combinations of
the 6 possible sequences in different patients. But this does not happen. What
is actually encountered is the sequence to be expected when the stomach is drawn
upward into a tubular HH by esophageal transtraction, i.e.
1.) Gastric mucosa histologically altered by ischemia due to constriction of
its blood supply in a hiatus designed to contain esophagus, not stomach, 2.)
Gastric cardiac mucosa, 3.) fundic mucosa.
There is a reason for the rising incidence of BE - the increasing use of air contrast examinations. Christensen and Lund have demonstrated that inflating the opossum esophagus causes reflex contraction of the LM(16) and this is certainly the case in man. When inflation is done at esophagoscopy, it will pull a HH through the hiatus and render a saccular HH tubular. The same is true radiologically if the examiner does an air esophagogram. The popularity of these examinations in recent years probably accounts for the current epidemic of BE. The recent reported cases are invariably illustrated in air contrast.(17)'(18),(19),(20),(21) Some of these show the "distal stricture" that is actually the less distensable sphincter area.
Lower esophageal rings are encountered with great frequency. Johnson et
al.(22) found them in 15-18% of 22,368
patients undergoing upper GI fluoroscopy. It is widely accepted that they occur
at the junction of gastric and esophageal mucosa. If gastric epithelium grew
orad into the esophagus, it would have to coat the LER. If any squamous epithelium
at all was transformed into columnar mucosa, the mucosa of the ring would be
involved. To my knowledge, no cases have been reported in which a LER was located
within or below a region of Barrett mucosa.
The demarcation line between squamous esophageal mucosa and columnar gastric
mucosa is sharp - at least as sharp as the ora serrata. Yet the entire esophagus
is exposed to acid pepsin in patients with reflux. What additional postulate
must be made to account for the sharp demarcation of BE? The interdigitations
of squamous and gastric mucosa which seem so convincing are simply what one
would expect at the ora serrata. The "dribbles" of squamous mucosa on to the
inferior surface of a LER when flattened out would appear to be interdigitations.
Ectopic gastric mucosa in the upper esophagus is not uncommon, occurring in
about 10% of the population,(23) usually at
the level of the thoracic inlet. It is generally agreed to be heterotopic. I
have not found any suggestion that it might be metaplastic. Endoscopically and
radiographically it bears no resemblance to BE. It presents as shallow saucer-like
depressions with slightly raised margins, not as cylinders of gastric mucosa.
Unlike BE, it is surrounded on all sides by squamous mucosa.
In my experience, the incidence of tubular hiatus hernias approximates the
reported incidence of BE. The appearance of a tube of stomach drawn through
the "die" of a small hiatus by esophageal shortening is identical with published
radiographs. The esophagus can easily shorten one third its length, retracting
a long tube of stomach through the hiatus whereapon the less distensable sphincter
will appear to be a "smooth stricture." These HHs may be persistent or they
may reduce. Science(24) quotes the
author of a 10-year study of BE as amazed that occasionally a BE spontaneously
reverts to normal. "It's the strangest thing we've ever seen . . . .", he said.
It would be strange indeed if a reverse metaplasia again reversed. Not so strange
if a HH reduced.
Although proving the diagnosis of BE is difficult, disproving it is easy: esophageal
peristalsis stops at the sphincter - there is no peristalsis in the gastric
fundus. Therefore, if peristalsis stops on reaching a tube of gut lined with
gastric mucosa, one can be certain the wave has encountered stomach lined with
gastric mucosa - not esophagus. Unfortunately, this test cannot be performed
endoscopically or with air esophagograms.
That said, it must be admitted that there is something unusual and significant
about such tubular HHs beside their shape. The persistent shortening of the
esophagus with its attendant reflux and the small hiatus that molds them and
constricts their blood supply without strangulating the stomach deserves separate
classification and analysis. If the tube of stomach is constantly above the
diaphragm, as may well be the case, the LM must be constantly shortened.
I have not attempted to examine the claim that the incidence of carcinoma is
greatly increased in such cases although this assertion deserves critical study
in view of the wide disparity in reported incidence. A Collis procedure, in
which a tube of stomach is formed into an artificial esophageal extension, duplicates
most of the characteristics of the postulated BE. It would be worth studying
a large series of such cases for the incidence of carcinoma.
Nothing is ever simple. There is some theoretical possibility that a congenitally
short esophagus may never have developed a squamous epithelial lining during
embryogenesis. Certainly, however, this would be a great rarity and not an affliction
of 12% of the GER population.
SUMMARY
Longitudinal shortening of the esophagus can be due to vagal stimulation, to hormonal influences, and to the direct effect of acid pH on the esophageal mucosa. It shortens reflexly from inflation of the organ at radiologic or endoscopic examination. All of these factors are at work in patients with supposed BE. They cause a tubular hiatal transtract that is mistaken by examiners for esophagus lined with gastric mucosa. The supposed metaplasia can vary up to 9 cm over a few weeks or vanish entirely as more or less stomach is pulled through the diaphragm. Metaplasia from unspecialized to more specialized tissue is unknown elsewhere and is not likely here where there is a perfectly reasonable explanation for the appearances. Microscopic diagnosis is illusory as it depends on the distance of the biopsy from the incisors or from a manometrically localized "sphincter" which may be a hiatal squeeze. The epithelium is indistinguishable from intestinal metaplasia of gastric mucosa - which indeed it is as demonstrated by its ability to take up technitium pertechnetate.
References
Last Updated July 31 2007 by David PJ Stiennon
1. 1. Winters, C Jr., Spurling, T.J., Chobanian, S.J., et al., Barrett's esophagus: a prevalent, occult complication of gastroesophageal reflux disease. Gastroenterology 92:118-24, 1987.
2. 2. Schnell, T., Sontag, S., Wanner, J., et al., (abstract), Gastroenterology 88:1576, 1985.
3. 3. Levine, MS., Barrett's esophagus: a radiologic diagnosis? AJR 151:433-438,1988.
4. . Shapir, J, DuBrow, W. and Frank, P., Barrett oesophagus: analysis of 19 cases. Brit. J. Rad. 58:491-3, 1985.
5. . Shirazi, S., Schulze-Dierieu, K., Custer-Hagen, T., et al., Motility changes in the opossum esophagus from experimental esophagitis. Dig. Dis. & Sci. 34:1668-76, 1989.
6. . Paterson, William G. And Kolyn, Donna, Esophageal shortening induced by short term intraluminal perfusion in opossum: a cause for hiatus hernia? Gastroenterology 107:1736-40, 1994.
7. . Gozzetti, G. Pelotti, V., Spangaro, M. et al., Pathophysiology and natural history of acquired short esophagus. Surgery 102:507-14, 1987.
8. Of doubtful validity - it is probably hiatal squeeze. If the supposed gastric mucosa hugs the scope it is certainly hiatal squeeze.
9. 8. Spechler, S.J. and Goyal, R.K., Barrett's Esophagus. NEJM, 315:362-71, 1986
10. 9. Kim, Suzy l., Waring, J. Patrick, Spechler, S.J., et al. Diagnostic inconsistencies in Barrett's esophagus, Gastroenterology 107:945-50. 1995.
11. 10. Gilchrist, Alison, Levin, Marc S., Carr, Robert F., Saul, Scott H., Herlinger, Hans and Laufer, Igor, Barrett's esophagus: diagnosis by double contract esophagography, AJR 150:97-102, 1987.
12. 11. Kweka, E.L., O'Neill, M., Cooney, C. And O'Sullivan, G., Imaging Barrett's esophagus. Clin. Rad. 38:415-8, 1987.
13. 14. It is said, however, that islands of gastric type mucosa form in the duodenum when it is colonized by H. pylori. This too is dubious as they may have pre-existed. It would be difficult to prove except in the unlikely event that the duodenum had been biopsied before and after the infection.
15. 12. Goldfarb, Richard, Ongseng, F, Finestone, H, et al. Letter, AJR, 152: 892, 1989.
16. 7. Christensen, James and Lund, Gordon F., Esophageal response to distention and electrical stimulation. J. Clin. Invest. 48:408-19, 1969.
17. 13. Chen, Y.M., Gelfand, D.W., Ott, D.J. and Wu, W.C., Barrett esophagus as an extension of severe esophagitis. AJR 145:275-81, 1981.
18. 14. Yulish, B.S, Rothstein, F.C. and Halpin, T.C., Jr. Radiographic findings in children and young adults with Barrett's esophagus. AJR, 148:353-57, 1987.
19. 15. Yulish, Barry S., Rothstein, Fred C. And Halpin, Thomas C. Jr., Radiographic findings in children and young adults with Barret's esophagus. AJR 148:354-7, 1987.
20. 16. Gilchrist, Alison, et al., op cit.
21. 17. Chernin, Mark M., Amberg, John R., Kogan, Fredrick J., Morgan, Tim R. And Sampliner, Richard E., Efficacy of radiologic studies in the detection of Barret's esophagus. AJR 147:257-60, 1986.
22. 18. Johnson, A.C,, Lester, P.D., Sudarsanam, D and Dunn, D., Esophagastrogastric ring: why and when we see it, and what it implies: a radiologic pathologic correlation. South. Med. J. 85:946-52, 1992.
23. 19. Ueno,J., et al., Ectopic gastric mucosa in the upper esophagus: detection and radiographic findings. Radiology 191:571-3, 1994.
24. 21. A new test gives early warning of a growing killer. Science 264:1847-8, 1994.